number of neutrons in sulphur|Sulfur Facts : Manila Sulfur is the 16th element in the periodic table and has a symbol of S and atomic number of 16. It has an atomic weight of 32.060 and a mass number of 32. Sulfur has sixteen . Level of strategy that includes business and corporate manager who translate statements of direction generated at corporate level into concrete objectives. Functional Level Level of strategy that includes managers of product, geographical, and functional areas and develop annual objectives and short-term strategies in areas including production .
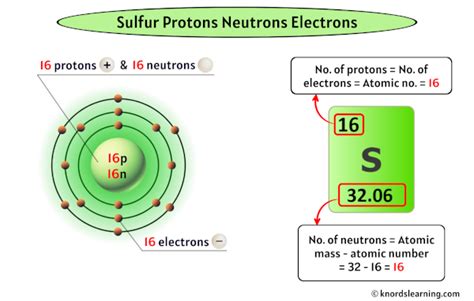
number of neutrons in sulphur,Neutron Number and Mass Number of Sulfur. Mass numbers of typical isotopes of Sulfur are 23; 33; 34; 36. The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. Neutron .number of neutrons in sulphur Sulfur Facts The total number of neutrons in the nucleus of an atom is called the neutron number of the atom and is given the symbol N. .

Sulfur. 16. 32.06. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. .
number of neutrons in sulphurSulfur is the 16th element in the periodic table and has a symbol of S and atomic number of 16. It has an atomic weight of 32.060 and a mass number of 32. Sulfur has sixteen .
Sulfur Facts Sulfur is the 16th element in the periodic table and has a symbol of S and atomic number of 16. It has an atomic weight of 32.060 and a mass number of 32. Sulfur has sixteen .In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sulfur (S). From the Periodic.
Natural sulfur is comprised of four stable isotopes: 32 S, 33 S, 34 S, and 36 S. Twenty one radioactive isotopes exist ranging from 26 S to 49 S. 32 S Sulfur-32 is a stable isotope containing 16 neutrons. .Number of protons: 16 p + Number of neutrons: 16 n 0: Number of electrons: 16 e-Number of Neutrons: 16. Classification: Non-metal. Crystal Structure: Orthorhombic. Density @ 293 K: 2.07 g/cm 3. Color: yellow. British Spelling: Sulphur. IUPAC Spelling: Sulfur. Atomic Structure. Number .The number of neutrons in the isotope can be calculated from its mass number, which is written as a superscript in a nuclear symbol. Mass Number = # of Protons + # of Neutrons 60 = 27 + # of Neutrons
The number of neutrons in sulfur is 16. The atomic mass number of sulfur (S) is 32. The atomic mass number is the sum of the protons and neutrons,. See full answer below.
Atomic Number of Sulfur. Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. The chemical symbol for Sulfur is S. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.Q. Sulphur has an atomic number 16 and a mass of 32. State the number of protons and neutrons in the nucleus of sulphur. Give a simple diagram to show the arrangement of electrons in an atom of sulphur.Mass Number = # of Protons + # of Neutrons. Mass Number = 1 + 2. Therefore, this particular atom of hydrogen will have a mass number of 3. Note that the mass number calculated in Example 2.4.1 2.4. 1 does not match the number underneath the elemental symbol and name for hydrogen on the periodic table. The formula for the number of neutrons is given as: Number of neutrons = Nearest whole number obtained by rounding up atomic mass – Number of protons. Rounding up 32.065, we get 32. Calculating the number of neutrons in the Sulfur atom ═ 32 ˗ 16 ═ 16. Hence, the number of neutrons in the Sulfur atom ═ 16. Now, we will .In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Sulfur (S). From the Periodic.
For all atoms with no charge, the number of electrons is equal to the number of protons. number of electrons = 30 number of electrons = 30. The mass number, 65, is the sum of the protons and the neutrons. To find the number of neutrons, subtract the number of protons from the mass number. number of neutrons = 65 − 30 .Because electrons have negligible mass, to account for the mass of the isotope, there must be 16 neutrons, 16 neutrally charged, massive, fundamental particles present in the sulfur nucleus. And thus finally, if we have the $$^{32}S^{2-}$$ ion, there are 18 electrons, 16 protons, and 16 neutrons.
Atomic Number: 16 Atomic Mass: 32.066 amu Melting Point: 112.8 °C (385.95 K, 235.04001 °F) Boiling Point: 444.6 °C (717.75 K, 832.28 °F) Number of Protons/Electrons: 16 Number of Neutrons: 16 Classification: Non-metal Crystal Structure: Orthorhombic Density @ 293 K: 2.07 g/cm 3 Color: yellow British Spelling: Sulphur IUPAC Spelling: Sulfur .
Sulfur ( 16 S) has 23 known isotopes with mass numbers ranging from 27 to 49, four of which are stable: 32 S (95.02%), 33 S (0.75%), 34 S (4.21%), and 36 S (0.02%). The preponderance of sulfur-32 is explained by its production from carbon-12 plus successive fusion capture of five helium-4 nuclei, in the so-called alpha process of exploding type .
The atomic number of sulphur is 16 and its atomic mass is 32. What is the number of neutrons in sulphur? View Solution. Q 2. Which of the following are true for an element? (i) Atomic number = number of protons + number of electrons. (ii) Mass number = number of protons + number of neutrons.
We would like to show you a description here but the site won’t allow us.Protons and Neutrons in Potassium. Potassium is a chemical element with atomic number 19 which means there are 19 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x . Sulfur is a abundant non metal that makes up 3% of the earth’s mass. It was discovered by Antoine Lavoisier in 1789 but in 1823 by the German Chemist Eilhard Mitscherlich obtained Sulfur crystals from cooling molten sulfur. Sulfur exists as allotropes (similar formulas but different structures) of a rhombic and monoclinic Sulphur crystals.
Given: Atomic number of Sulphur (S) = 16. Mass number of Sulphur (S) = 32. Formula used: Atomic number = Number of electrons = Number of protons; Mass number = Number of protons + Number of neutrons; Formation of ion formed from Sulhur atom: The atomic number of Sulphur is 16.Thus, its electronic configuration is (2, 8, 6).; A .First, to find the number of protons, we need to realize that the neutral atom had 53 electrons because it is the additional one electron that makes it a 1- anion. Now, because the atom has 53 electrons, it must also have 53 protons, and to find the number of neutrons we subtract this from the mass number. # n = A – # p = 127 – 53 = 74 .

The mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average. Isotopes Atoms of the same element with different numbers of neutrons. CAS numberSulfur, also spelled as sulphur, is a very important element in today's world. Its most important use is in the manufacture of sulfuric acid (H 2 . The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope. .
number of neutrons in sulphur|Sulfur Facts
PH0 · Sulfur Facts
PH1 · Sulfur (S)
PH2 · Sulfur (S)
PH3 · Sulfur
PH4 · Protons, Neutrons, Electrons for Sulfur (S, S2
PH5 · How to find the Number of Protons, Electrons,
PH6 · Chemical Elements.com
PH7 · 2.4: Neutrons: Isotopes and Mass Number Calculations